How Much Voltage Is Required For Electrolysis Of Water
When present the applied potential must be increased making it possible for a different reaction to occur in the electrolytic cell.
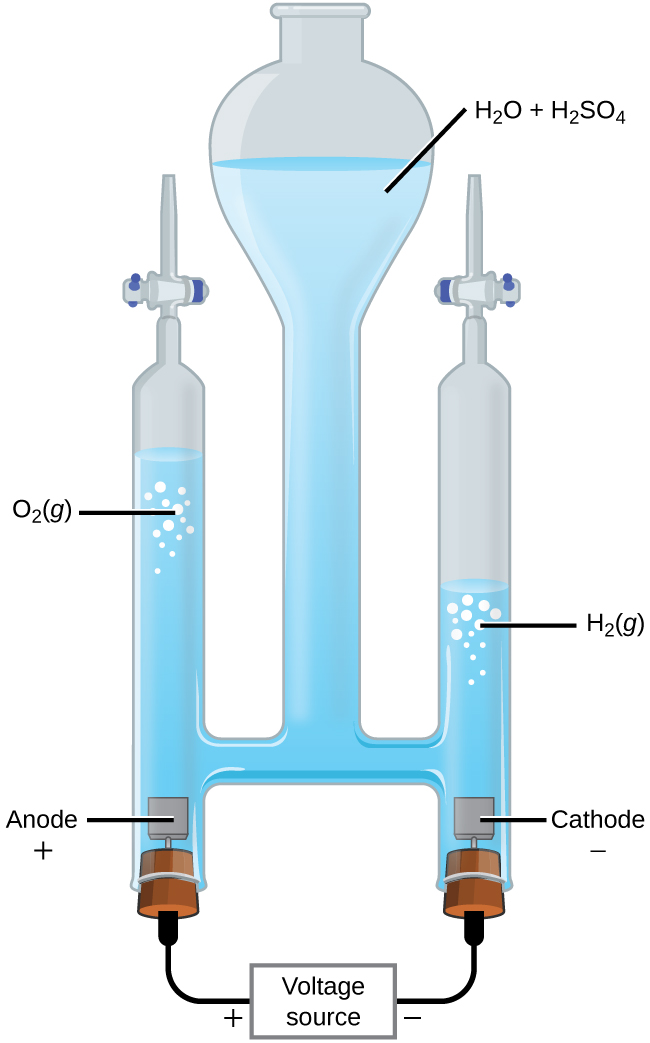
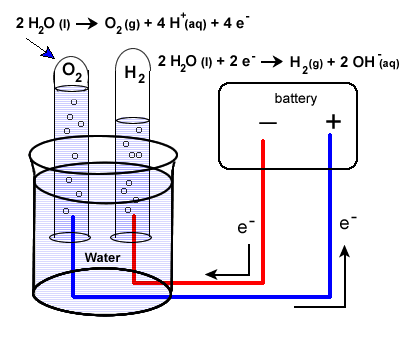
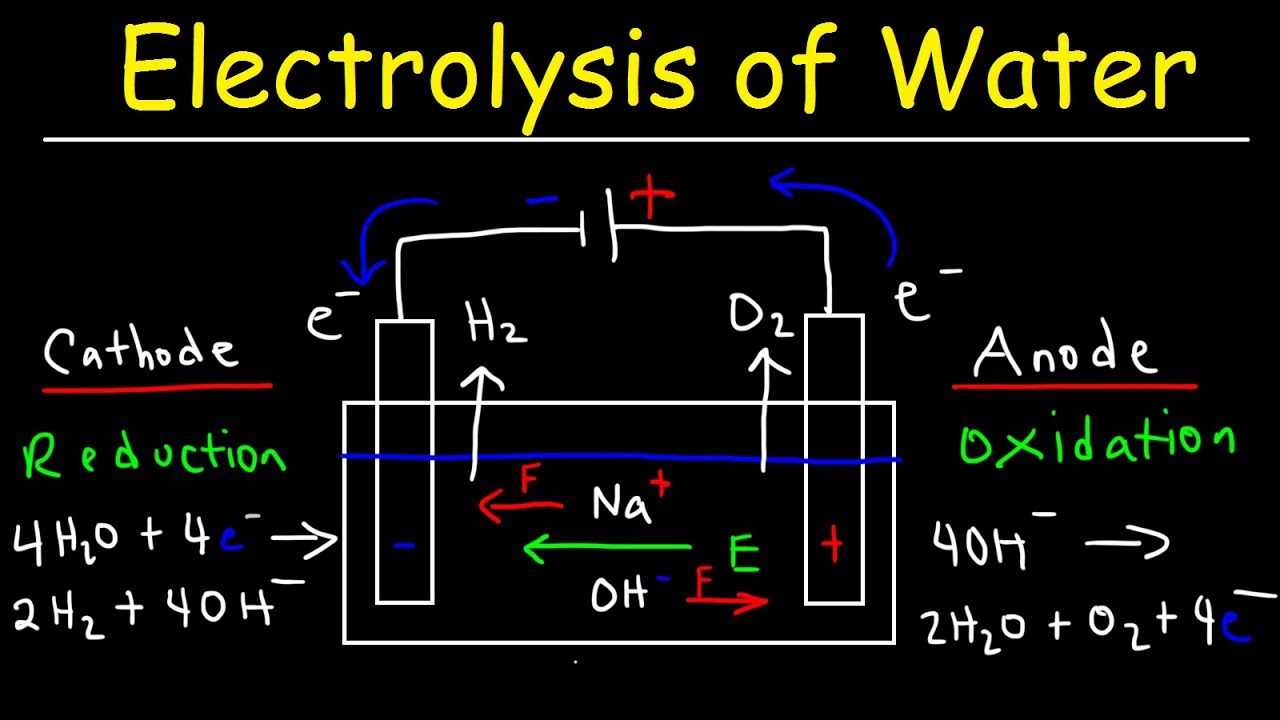
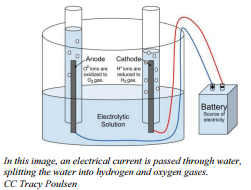
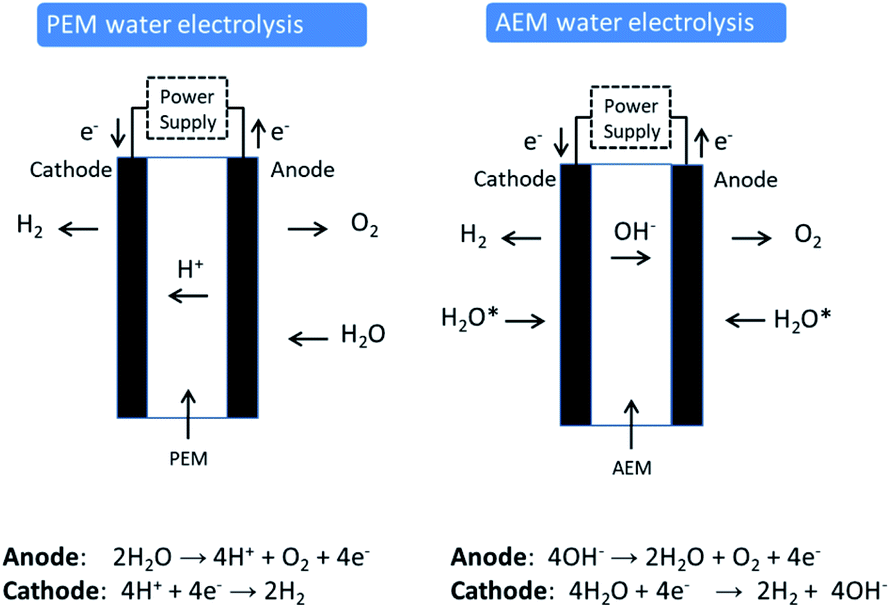
How much voltage is required for electrolysis of water. Electrolysis is a leading hydrogen production pathway to achieve the Hydrogen Energy Earthshot goal of reducing the cost of clean hydrogen by 80 to 1 per 1 kilogram in 1 decade 1 1 1. This example explains why the process is called electrolysisThe suffix -lysis comes from the Greek stem meaning to loosen or split up. To control the amount of substances produced at the anode and cathode the current needs to be controlled.
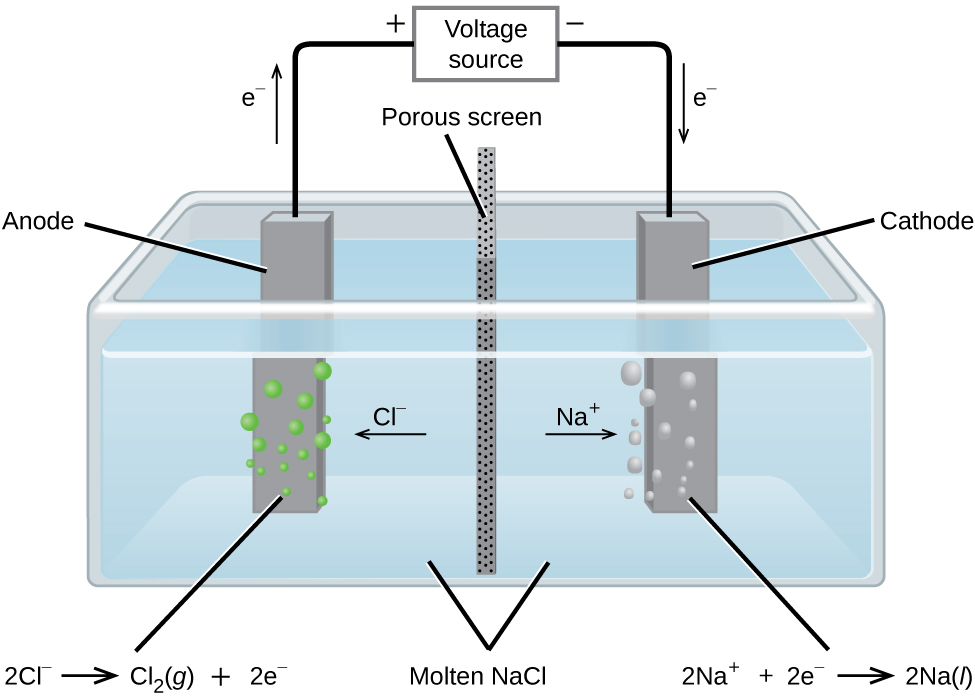
The potential required to oxidize Cl-ions to Cl 2 is -136 volts and the potential needed to reduce Na ions to sodium metal is -271 volts. It depends on how you do it if it will take that much or more if you dont use the correct. Answer 1 of 3.
Producing 25 mL of Hydrogen using 230 V takes 90 seconds with Gold and tap water. Electrolysis of Water By providing energy from a battery water H 2 O can be dissociated into the diatomic molecules of hydrogen H 2 and oxygen O 2This process is a good example of the the application of the four thermodynamic potentials. It wont be long maybe 20 minutes.
Even this smoothing capacitor is not needed. Ideally 39 kWh of electricity and 89 liters of water are required to produce 1 kg of hydrogen at 25 C and 1 atmosphere pressure. However in practice you need more than this for two reasons.
Overpotential is the difference between the theoretical half-reaction reduction potential and the actual voltage required. The water itself becomes flammable. Calculation for 1 kg of water 5555 moles.
Hydrogen electrolysis occurs anytime a voltage is applied to water even when other molecules are dissolved into itThis makes solution electrolysis fairly dangerous because water is composed of hydrogen and oxygen. Periods l Content Standard. The energy required to split water into hydrogen and oxygen by electrolysis is 260 kJ per mole of water.